How Complexity Thinking Can Transform Clinical Trials
A Design Approach to Pharmaceutical Innovation
Executive Summary: Strategic Advantages for Pharmaceutical Leadership
- Current challenge: Clinical trials cost $1+ billion per drug while diverse recruitment mandates intensify, threatening bottom-line performance and regulatory approval timelines
- Strategic opportunity: Design thinking methodology delivers 30-50% faster recruitment, 40% fewer amendments ($500,000 savings each), and improved regulatory compliance through systematic patient-centred approaches
- Competitive advantage: Early adopters are gaining first-mover benefits through accelerated time-to-market, increased patent-protected revenue periods, and preferred status with regulators focused on trial diversity
- Implementation path: Begin with Phase III and market surveillance studies in community settings, building research capability beyond traditional centres while demonstrating immediate ROI
The companies that strategically implement design thinking in clinical operations now will establish sustainable competitive advantage as regulatory requirements continue to tighten and patient expectations evolve.
The Costly Reality of Drug Development
David, a senior research executive at a major pharmaceutical company, faces a challenge familiar to many in the industry. He's overseeing the development of a promising compound that's crucial to his company's pipeline. Regulatory approval is high priority after significant investment, but he must conduct trials that are both cost-efficient and compliant with increasingly stringent FDA guidelines, including diverse population requirements. His trial network is limited to a few urban research centres. Speed is critical—delays could affect approval timelines and market position.
This scenario plays out daily across the pharmaceutical industry, where development timelines stretch to 15 years and costs reach over $1 billion per approved drug [1]. For medical devices, costs range from $31-94 million depending on complexity [2]. With patents lasting only 20 years, these extended timelines significantly impact return on investment.
"The pharmaceutical industry's development model is not sustainable. No other sector could survive such a dysfunctional way of doing business."
What's particularly striking is that while other highly regulated industries like automotive and aerospace have continuously improved their development processes, pharmaceutical timelines have actually lengthened. As someone who worked in both automotive design at Jaguar and healthcare innovation, I've observed this contrast firsthand.
Why Clinical Trials Are Stuck in the Past
Clinical trials suffer from multiple interconnected challenges:
- Centralisation: Research occurs primarily in academic and industry hubs, excluding diverse populations
- Fragmentation: Unlike the integrated value chains in aerospace or automotive, healthcare stakeholders lack structural connectivity
- Outsourcing complexity: The proliferation of Contract Research Organisations (CROs) has inadvertently added coordination challenges
- Regulatory hurdles: Multiple regulatory authorities worldwide create complicated approval pathways
The PPD survey of 150 drug developers found that patient enrolment challenges—including diversity and retention—topped their concerns (55%), followed by trial complexity (51%) and regulatory hurdles (46%) [3].
These challenges aren't isolated problems—they form a complex system with interdependent elements. When pharmaceutical companies treat them as separate issues to be solved sequentially, they miss the systemic nature of the challenge.
Complexity Thinking: Understanding the System
Complexity thinking provides valuable insights into how clinical trial systems behave, but it primarily offers diagnosis rather than treatment. It helps us understand why:
Patient recruitment challenges persist despite detailed planning
Protocol amendments multiply throughout the trial lifecycle
Diverse population inclusion remains difficult despite clear mandates
Stakeholder coordination breaks down across organisational boundaries
Complexity science reveals that clinical trials are non-linear systems where small changes can have disproportionate effects, and where solutions must consider the entire ecosystem rather than isolated components.
Design Thinking: Transforming Understanding into Action
While complexity thinking provides analytical insights, design thinking offers a framework for practical action. As Mads Øvlisen, former CEO of pharmaceutical company Novo Nordisk A/S, noted: "At its best, design solves a problem so well that no one even notices that there ever was a problem" [4].
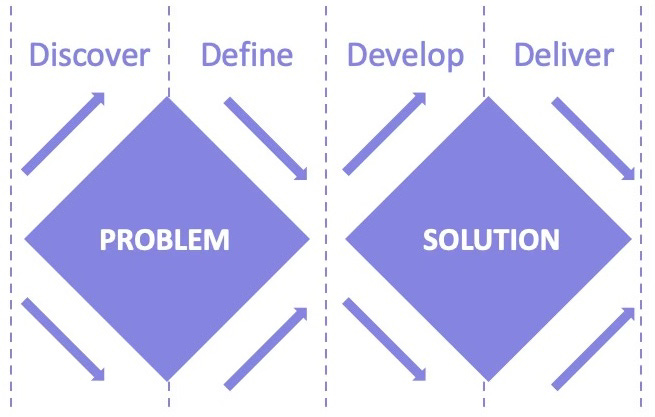
The UK Design Council's Double Diamond model provides a powerful framework for clinical trial transformation:
The Double Diamond consists of four phases:
Discover: Research to understand the problem space
Define: Synthesize findings to identify core challenges
Develop: Generate potential solutions
Deliver: Implement and refine solutions
What's critical about this model is that it dedicates equal effort to understanding the problem (first diamond) as to creating solutions (second diamond). In pharmaceutical development, companies typically underinvest in discovery and definition, rushing to development—with predictable consequences.
Practical Applications in Clinical Trials
Design thinking transforms clinical trials through several practical applications:
1. Empathy and Understanding of Diverse Patient Needs
Design Tools: Personas, patient journey mapping
By developing detailed patient personas and mapping their journeys, companies gain deep insights into barriers to participation. This reveals why traditionally underrepresented groups don't participate and what would enable their inclusion.
2. Co-Design and Community Engagement
Design Tools: Ethnography, stakeholder workshops
Rather than designing protocols in isolation, pharmaceutical companies can engage community healthcare providers, patient advocates, and diverse stakeholders in protocol development. This identifies practical barriers before implementation and builds trust with historically excluded communities.
3. Inclusive Recruitment Strategies
Design Tools: Rapid prototyping, community testing
Prototype recruitment strategies can be tested in small-scale community settings before full implementation, identifying what works for specific populations. This iterative approach allows companies to refine methods before committing resources to full-scale recruitment.
4. Accessible Trial Locations and Processes
Design Tools: Decentralised trial design, mobile capabilities
Building research capability in community clinics rather than relying solely on academic centres makes participation accessible to broader populations. This decentralisation can begin with Phase III trials and market surveillance studies, gradually building community research capability.
Building community research capacity requires a phased approach:
Assessment phase: Evaluate existing capabilities, staff experience, and infrastructure needs
Training and certification: Provide Good Clinical Practice (GCP) training to clinic staff, with mentorship from experienced research sites
Infrastructure development: Start with minimal technology and documentation requirements, gradually building sophisticated capabilities
Paired research models: Partner community clinics with academic centres for early support, gradually transitioning to independent research operations
Sustainable funding models: Design trial compensation that accounts for the true cost of research in resource-constrained settings
This graduated approach allows pharmaceutical companies to expand their research footprint while community providers build sustainable research programs that continue beyond individual trials.
Bridging Understanding and Action
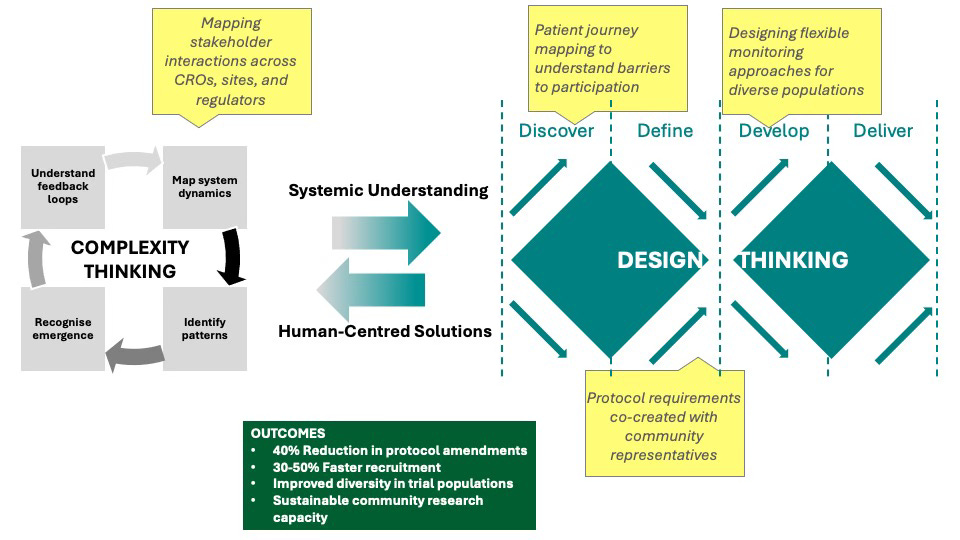
As shown in the figure below, complexity thinking provides the analytical foundation to understand clinical trial challenges systemically, while design thinking offers a structured methodology to transform these insights into patient-centred solutions. This integration creates a powerful framework that bridges understanding and action, resulting in measurable improvements in trial efficiency, diversity, and community engagement.
The Double Diamond model, illustrated earlier, provides the practical framework for implementing this integrated approach.
Case Study: Design Thinking in Action
When a major pharmaceutical company needed to recruit diverse participants for a cardiovascular disease trial, they took a design thinking approach:
Discovery phase: Instead of relying solely on clinical data, researchers spent time in community settings understanding barriers to trial participation. They discovered transportation difficulties, work schedule conflicts, and mistrust were primary barriers.
Definition phase: The team co-created trial requirements with community representatives, focusing on what would make participation possible rather than just scientifically ideal.
Development phase: The protocol incorporated evening appointments, transportation support, and community-based monitoring to reduce hospital visits. Local healthcare providers were involved as co-researchers rather than just referral sources.
Delivery phase: The trial completed enrollment 40% faster than comparable studies, achieved representation matching disease prevalence demographics, and required zero major protocol amendments.
The Financial Case for Design Thinking
The business case for implementing design thinking is compelling:
Reduced development costs: By improving trial design through stakeholder input early in the process, companies avoid costly protocol amendments averaging $500,000 per change
Faster time-to-market: Decentralised trials with improved recruitment can reduce enrolment periods by 30-50%, potentially worth hundreds of millions in additional patent-protected market time
Higher success rates: Better understanding of diverse patient needs leads to more effective therapies and fewer late-stage failures
Regulatory compliance: Meeting FDA diversity requirements more efficiently prevents approval delays costing $1-2 million per day in lost revenue
Equity Outcomes Beyond Representation
While improved trial diversity meets regulatory requirements, the equity implications of design thinking extend much further. When community providers develop research capabilities, they gain:
Enhanced clinical expertise that benefits all patients, not just trial participants
New revenue streams that strengthen financial sustainability in underserved areas
Improved standard of care as evidence-based practices from research protocols influence routine care
Community health literacy as research participation normalises clinical innovation
These structural benefits address healthcare disparities at their root, creating lasting impact beyond any single trial.
AI as a Design Thinking Enabler
As explored in my previous article on AI in healthcare, artificial intelligence can exponentially enhance design thinking approaches. Several promising applications are emerging in the clinical trials space:
Patient journey simulation: Advanced AI platforms can simulate how different patient populations might experience a protocol, identifying participation barriers before implementation. In recent diabetes trials, this approach has helped researchers modify visit schedules based on predicted transportation challenges for rural participants.
Community mapping: Geographic AI analysis can identify optimal locations for decentralised trial sites based on disease prevalence, demographics, and healthcare access patterns—improving diversity without extending recruitment timelines. This data-driven approach replaces intuition with evidence when selecting community research partners.
Protocol optimisation: Machine learning systems analysing previous trial designs against performance metrics can suggest protocol modifications that reduce amendments while improving patient retention. One mid-sized pharmaceutical company reported 35% fewer amendments in their respiratory studies after implementing this approach.
Real-time adaptation: Adaptive trial platforms using continuous monitoring can suggest protocol adjustments, successfully rescuing trials experiencing high dropout rates by modifying assessment procedures based on patient feedback patterns.
These applications demonstrate how AI amplifies design thinking's impact, enabling pharmaceutical companies to implement human-centred approaches at scale across global research programs while maintaining scientific rigour.
Navigating Organisational Change
Implementing design thinking in pharmaceutical organisations requires more than methodological adoption—it demands cultural transformation. Traditional pharmaceutical R&D culture emphasises scientific rigour and regulatory compliance, sometimes at the expense of patient experience and operational efficiency.
Successful transformation leaders have employed several strategies:
Executive sponsorship: Securing visible support from senior leadership who consistently reinforce the value of design approaches
Cross-functional teams: Establishing dedicated teams that bring together clinical, regulatory, operational, and patient advocacy perspectives
Success metrics: Creating new evaluation criteria that reward patient-centred innovation alongside scientific validity
Capability building: Investing in design thinking training across departments rather than isolating it within innovation teams
Parallel processes: Running traditional and design-led approaches simultaneously until the organisation builds confidence in new methodologies
What if pharmaceutical companies establishing a "Clinical Design Studio" that paired designers with clinical scientists, gradually expanding from a small innovation team to influencing the entire development process over three years.
Implementing Design Thinking: A Practical Roadmap
For pharmaceutical executives considering design thinking, a stepped implementation approach reduces risk while building organisational capability:
Begin with a pilot: Select an upcoming Phase III trial with diversity requirements and apply design thinking to recruitment and retention strategies
Build internal capability: Develop a cross-functional team with training in design methods and co-design facilitation
Engage community partners: Identify 3-5 community clinics in underserved areas and develop partnership models enabling research participation
Measure and learn: Track key metrics including recruitment speed, participant diversity, and protocol amendments to demonstrate ROI
Scale successful approaches: Apply lessons to additional therapeutic areas and earlier phase trials
Addressing Common Concerns
Executives may have legitimate concerns about implementing design thinking:
"We don't have time for this additional process"
Design thinking front-loads effort to prevent costlier delays later. By spending adequate time in the "Problem" diamond, companies avoid expensive amendments during the "Solution" phase.
"Regulatory requirements don't allow for this flexibility"
Regulatory agencies increasingly encourage patient-focused drug development. The FDA's Patient-Focused Drug Development initiative specifically calls for greater input from diverse patient populations, making design thinking aligned with regulatory direction.
"Our scientific teams aren't trained in design methods"
Expertise in design can be developed through targeted training or partnerships with design specialists. The investment yields returns across multiple trials and therapeutic areas.
Conclusion: Transforming the Industry
The pharmaceutical industry faces unprecedented challenges that require new approaches. By combining complexity thinking's analytical power with design thinking's practical methodologies, companies can transform clinical trials from expensive, time-consuming processes into efficient, inclusive systems that deliver better treatments to more diverse populations.
This transformation benefits all stakeholders:
- Patients gain access to potentially life-saving trials regardless of location or background
- Healthcare providers in community settings develop research capabilities
- Pharmaceutical companies reduce costs while improving regulatory compliance
- Regulatory bodies achieve their diversity mandates through practical implementation
For those leading pharmaceutical organisations, the opportunity is clear: design thinking offers a proven methodology to navigate complexity, reduce costs, and bring life-changing treatments to market faster.
Questions for Discussion
How has your organisation addressed diversity requirements in clinical trials? What approaches have worked or failed?
What barriers do you see to implementing design thinking methodologies in your clinical research operations?
How might community healthcare providers become more active participants in your clinical trial ecosystem?
---
In my next article, "Why Healthcare Innovation Fails: The System Wasn't Designed for It," I’ll explore how healthcare's structural characteristics create barriers to innovation that even the most promising technologies and methodologies struggle to overcome. We'll examine why healthcare resists change more than other sectors and identify the design principles needed to build systems that can evolve and adapt to meet emerging challenges. We will also explore how design relates to different types of innovation and which are most relevant for transformation. This will further develop our understanding of how design thinking and complexity approaches can transform not just clinical trials, but healthcare delivery as a whole.
References
[1] Research and Development in the Pharmaceutical Industry | Congressional Budget Office [WWW Document], 2021. URL https://www.cbo.gov/publication/57126
[2] Sertkaya, A., DeVries, R., Jessup, A., Beleche, T., 2022. Estimated Cost of Developing a Therapeutic Complex Medical Device in the US. JAMA Network Open 5, e2231609. https://doi.org/10.1001/jamanetworkopen.2022.31609
[3] Moore, T.J., Heyward, J., Anderson, G., Alexander, G.C., 2020. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015–2017: a cross-sectional study. BMJ Open 10, e038863. https://doi.org/10.1136/bmjopen-2020-038863
[4] In New Innovation Actors by Dansk Design Center - Issuu [WWW Document], 2014. URL https://issuu.com/dansk\_design\_center/docs/rap\_innovation\_actors\_gb (accessed 2.21.25).
[5] Dukart, H., n.d. Emerging from disruption: The future of pharma operations strategy | McKinsey [WWW Document]. URL https://www.mckinsey.com/capabilities/operations/our-insights/emerging-from-disruption-the-future-of-pharma-operations-strategy
[6] Rees, H., 2011. Supply Chain Management in the Drug Industry. Wiley.
[7] Kimmitt, R., n.d. R&D Time and Success Rate [WWW Document]. Knowledge Portal. URL https://www.knowledgeportalia.org/r-d-time-and-success-rate
[8] Burrows, A., n.d. The Trends and Challenges of Clinical Outsourcing [WWW Document]. Drug Discovery from Technology Networks. URL http://www.technologynetworks.com/drug-discovery/articles/the-trends-and-challenges-of-clinical-outsourcing-184345
[9] Nelson, H., 2012. The Design Way: Intentional Change in an Unpredictable World., 2nd ed. MIT Press.
[10] The Double Diamond - Design Council [WWW Document], n.d. URL https://www.designcouncil.org.uk/our-resources/the-double-diamond/